Water disinfection serves as the crucial final barrier between treated wastewater and disease outbreaks in communities. Harmful microorganisms in treated wastewater could trigger serious health problems in humans and animals without proper disinfection. The process targets and eliminates dangerous pathogens, making it the most vital safeguard in our water treatment system.
The final step in wastewater treatment involves disinfection after primary, secondary, and sometimes tertiary processes. This piece explores water treatment disinfection methods of all types. You’ll learn about how different water disinfection chemicals destroy pathogens and what affects their performance. Safety regulations ensure water quality stays consistent. The process’s significance goes beyond drinking water and helps reduce waterborne diseases in various industries.
What Disinfection Means in Wastewater Treatment

Wastewater management’s disinfection process stands out as a vital step that neutralizes harmful microorganisms before water returns to the environment. This process targets pathogenic organisms that cause diseases like cholera, typhoid, hepatitis, and various bacterial, viral, and parasitic infections.
Difference Between Disinfection and Sterilization
People often mix up disinfection and sterilization, but these are two different approaches to microbial control. Disinfection aims to reduce harmful microorganisms to safe levels. Sterilization, on the other hand, removes all microorganisms, even the harmless ones. This difference has real practical implications.
Let’s examine water disinfection methods. Here’s what you need to know about disinfection:
- It reduces pathogenic microorganisms to safe levels but doesn’t eliminate all microbial presence
- We target bacteria, viruses, and protozoa that cause disease
- Chemical processes work best, especially for water treatment
- Resistant bacterial spores might survive, unlike with sterilization
Sterilization works differently:
- It kills all but none of these microorganisms, including spores
- You need intensive processes like pressurized steam, hydrogen peroxide gas, or radiation
- Medical instruments and laboratory equipment use this method rather than water treatment
On top of that, the CDC breaks down disinfection into different levels. High-level disinfectants kill almost all microorganisms except large numbers of bacterial spores. Intermediate-level ones eliminate mycobacteria, most viruses and fungi. Low-level disinfectants destroy most vegetative bacteria and some viruses and fungi.
Why Disinfection is the Final Step in Treatment
Disinfection comes last in the wastewater treatment sequence, and with good reason too. Placing disinfection at the end will give maximum effectiveness to the disinfection chemicals. Earlier stages—primary, secondary, and sometimes tertiary—remove organic materials and suspended solids that might interfere with the process.
This final protective barrier kicks in before water returns to the environment. Previous treatment steps remove most contaminants, which helps disinfection target remaining pathogens more effectively. This order prevents waterborne diseases that earlier treatments don’t deal very well with.
Timing plays a vital role because:
- Previous treatments remove substances that might create harmful by-products with disinfectants
- Better disinfection happens after removing suspended solids, which can shield microorganisms
- You can control contact time requirements (usually two hours for chlorination) better at this final stage
Getting completely sterile water would be ideal but isn’t cost-effective for most wastewater treatment facilities. The main goal focuses on meeting regulatory standards and making water suitable for environmental discharge or reuse. This means targeting microorganisms that risk public health rather than eliminating all microbial life.
Key Disinfection Methods Used in Water Treatment
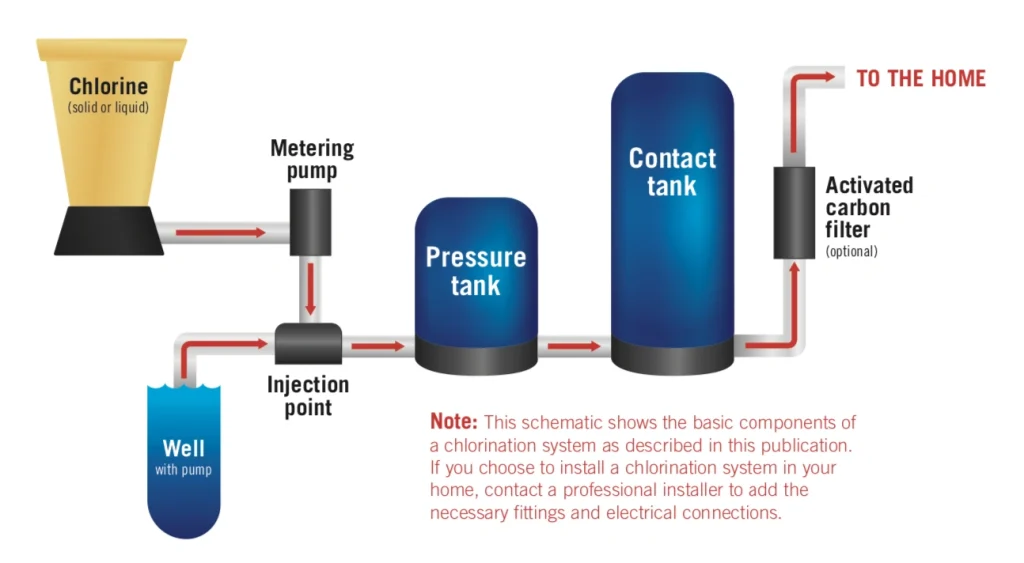
Modern water treatment facilities use different disinfection technologies. Each technology kills harmful pathogens in its own way. These methods work differently against specific microorganisms and come with their own costs and environmental effects. Water treatment facilities choose the best disinfection strategy by understanding the science behind each method.
Chlorination and Its By-Products
Chlorination stands as the most common water disinfection method worldwide. It’s affordable and protects water even after treatment. Chlorine creates hypochlorous acid (HOCl) and hypochlorite ions (OCl-) when added to water. These compounds get inside microbial cells and stop vital functions. This kills bacteria and viruses by shutting down the enzymes they need to survive.
Water treatment plants use chlorine in three forms:
- Chlorine gas (most common in larger facilities)
- Sodium hypochlorite (liquid form)
- Calcium hypochlorite (solid tablets, preferred for smaller systems)
Chlorination works well but creates some serious concerns about disinfection by-products (DBPs). These form when chlorine meets natural organic matter in water. The result? Potentially harmful compounds like trihalomethanes (THMs) and haloacetic acids (HAAs). The EPA limits THMs to 80 μg/L and HAAs to 60 μg/L in drinking water. Scientists have found chloroform, dichloroacetic acid, trichloroacetic acid, and dichloronitrile as the main components of these DBPs. The largest longitudinal study links these compounds to higher cancer risks.
Ultraviolet (UV) Radiation for DNA Disruption
UV disinfection takes a physical approach instead of using chemicals. This method uses electromagnetic radiation (usually at 254 nm wavelength) to break down microorganisms’ genetic material. UV light first passes through the pathogen’s cell wall. The radiation then damages nucleic acids by creating thymine dimers—unusual bonds between nearby thymine bases in DNA. These changes stop microorganisms from multiplying, which leads to their death.
UV treatment works best between 200-280 nm (UV-C spectrum), with peak effectiveness at 260-265 nm. UV-C proves especially powerful because it matches DNA and RNA’s maximum UV absorption. This technology kills chlorine-resistant organisms like Cryptosporidium. It needs just 20-30 seconds to eliminate 99.9% of total coliforms.
Ozonation and Cell Wall Oxidation
Ozonation uses one of nature’s strongest oxidizers—ozone (O₃). Treatment plants make ozone on-site through corona discharge in oxygen or dry air. Ozone quickly breaks down in water to create free radicals, including hydrogen peroxy (HO2) and hydroxyl (OH). These radicals pack an incredible oxidizing punch.
The process works by destroying the cellular wall through direct oxidation. This causes vital cellular components to leak out. Cells then break apart through protoplasmic oxidation. Ozone treatment proves highly effective—it kills up to 99% of E. coli bacteria and outperforms chlorine against viruses and bacteria.
Ozone treatment offers speed as a major advantage. It needs only 10-30 minutes to work. Unlike chlorine, it doesn’t leave harmful residuals because ozone quickly turns into oxygen. The biggest problem? This method needs complex equipment like ozone generators, contact chambers, and air dryers. This makes it much more expensive than regular chlorination systems.
Water treatment professionals must think about several factors when designing effective systems. These include target pathogens, water quality, operational costs, and environmental impact. Each disinfection method brings its own strengths and challenges to the table.
Factors That Influence Disinfection Effectiveness
Water disinfection success depends on several variables that can make it more or less effective. The most powerful disinfectants might not work as expected if these key factors aren’t handled properly during system design and operation. Treatment facility operators can optimize their processes in different conditions by understanding these influences.
Impact of pH, Temperature, and Suspended Solids
Water’s physical and chemical properties change how well disinfection works. The pH levels directly affect antimicrobial activity. Quaternary ammonium compounds and glutaraldehyde work better at higher pH, while hypochlorites and iodine are more effective in acidic conditions. pH changes both the disinfectant molecule structure and the microbial cell surface.
Temperature is crucial to disinfection kinetics. Most disinfectants become more effective as temperatures rise. High temperatures can break down some disinfectants and reduce their germ-killing power. Cold weather operations might need adjusted dosing or contact times because low temperatures slow down reaction rates.
Suspended solids create the biggest challenge among water quality factors. These particles protect microorganisms from disinfectants by physically shielding them. Bacteria hidden in particles stay safe from chemical disinfectants. UV disinfection faces an even bigger problem as solids block light and reduce effectiveness. Research shows that killing power drops tenfold when suspended solids increase from 6 to 85 mg/L.
Microorganism Type and Resistance
Different pathogens resist disinfection in various ways. Bacterial spores are the toughest to kill, followed by mycobacteria, gram-negative bacteria, and cocci. This resistance comes from built-in protection. Spores have protective coats and cortexes that block disinfectants, while mycobacteria’s waxy cell walls keep disinfectants out.
A microbe’s age changes how easily it dies. Young bacteria die more easily than older ones. Mature bacteria develop polysaccharide shells over their cell walls for better defense. To cite an instance, 10-day-old bacteria need 30 minutes of contact with 2.0 mg/L chlorine, while 1-day-old bacteria of the same species die in just 1 minute.
Required Contact Time for Each Method
Contact time forms the foundation of effective disinfection. Each method needs specific exposure times. Chlorination needs 30 minutes at 3 mg/L residual concentrations to kill bacteria and viruses. This time must go up in tough conditions like low temperature or high pH.
UV disinfection’s success depends on light intensity and exposure time. The needed dosage rises with flow rate. Ozonation works faster than chlorination and needs only 10-30 minutes of contact time.
Water quality affects required contact time. Organic materials use up active ingredients, leaving less to kill pathogens. This especially affects chlorine and iodine disinfectants. Treatment facilities must mix properly and design contact chambers with rounded corners. This prevents dead spots and short-circuiting.
Regulatory Standards for Disinfection Compliance
Federal and international regulations set long-standing standards for water disinfection effectiveness to protect public health. These regulatory frameworks ensure consistent safety in water treatment facilities throughout distribution networks.
EPA and WHO Microbial Limits
The Environmental Protection Agency (EPA) requires specific inactivation or removal levels for various pathogens in drinking water. The National Primary Drinking Water Regulations state that systems using surface water must achieve 99.9% removal/inactivation of Giardia lamblia and 99.99% removal/inactivation of viruses. The EPA believes proper handling of Giardia and viruses will lead to Legionella control, though no specific limit exists. The World Health Organization (WHO) guidelines serve as the foundation for water quality standards worldwide. These guidelines support locally relevant standards and preventive risk management approaches from catchment to consumer.
Disinfectant Residual Requirements
Water treatment facilities must maintain both minimum and maximum disinfectant residual levels. The maximum residual disinfectant level (MRDL) for chlorine is 4.0 mg/L. Each disinfectant type has different minimum required levels. Free chlorine needs at least 0.2 mg/L, while total chlorine (chloramines) requires a minimum of 0.5 mg/L. Residual disinfectants play three significant roles:
- Protection against microbial contaminants
- Indication of distribution system issues
- Prevention of bacterial growth within distribution systems
Monthly sampling based on system-specific plans determines compliance with disinfectant residual requirements. Smaller systems that collect fewer than 40 samples monthly can have no more than one sample below minimum levels for two consecutive months.
Monitoring for Fecal Coliform and E. coli
Total coliforms, fecal coliforms, and E. coli are the main indicators of possible pathogenic contamination. The EPA’s Maximum Contaminant Level Goal shows zero tolerance for total coliforms, with an actual compliance level of 5.0%. E. coli detection requires immediate follow-up testing. Additional analysis for fecal coliforms or E. coli becomes mandatory if any sample contains total coliforms. E. coli monitoring methods can detect one organism per sample volume tested. So, any confirmed E. coli presence suggests potential fecal contamination and possible health risks.
Challenges and Innovations in Disinfection
Water treatment facilities today face new challenges that require state-of-the-art solutions. These groundbreaking approaches help solve ongoing problems with disinfection systems in facilities worldwide.
Managing Disinfection By-Products (DBPs)
When disinfectants react with organic materials, they create harmful compounds such as trihalomethanes (THMs), haloacetic acids (HAAs), chlorite, and bromate. These DBPs can cause serious health problems, including cancer and reproductive issues. The EPA’s Disinfectants and Disinfection Byproducts Rule sets strict limits at 0.08 mg/L for THMs and 0.06 mg/L for HAA5 in drinking water.
Treatment facilities can reduce DBP formation through these methods:
- Enhanced coagulation removes organic matter before disinfection
- Alternative disinfectants like UV or ozone replace THM-forming agents
- Chlorination happens downstream after removing organic matter
- pH level adjustments minimize HAA formation
Advanced Oxidation Processes (AOPs)
AOPs show great promise in water disinfection by using powerful hydroxyl radicals that destroy stubborn pathogens and pollutants. These methods work well against pharmaceuticals, pesticides, and industrial contaminants when standard approaches fall short.
Popular AOP technologies combine UV/H₂O₂, use ozonation with peroxide, employ Fenton reactions, photocatalysis, and electrochemical oxidation. AOPs stand out from traditional methods because they kill pathogens and eliminate DBPs simultaneously. The market for chlorine water disinfectants should reach $4.78 billion by 2030, growing at 3.3% CAGR from 2024-2030.
Real-Time Monitoring and Sensor Integration
Modern disinfection systems now use advanced sensors that constantly measure disinfectant levels. Amperometric sensors enable live monitoring of free chlorine, total chlorine, chlorine dioxide, ozone, and other oxidants. These sensors measure electron flow that corresponds to disinfectant concentration.
Fluorescence sensors add more capabilities to monitor water quality during treatment. They offer several key benefits:
- Better process optimization reduces secondary pollution
- Smart dosing prevents excess disinfectant use
- Quick detection spots disinfection problems early
- Analytical insights reduce unnecessary sampling
Conclusion
Water disinfection serves as the last critical barrier that protects public health from waterborne pathogens. This piece explores how different disinfection methods eliminate harmful microorganisms before treated wastewater returns to our environment. Chlorination remains the standard method because it’s economical and provides residual protection, though concerns exist about disinfection by-products. UV radiation offers a chemical-free alternative by disrupting pathogen DNA, while ozonation provides powerful oxidation with fewer harmful residuals.
The success of disinfection depends on several key factors. Treatment effectiveness changes based on temperature, pH levels, suspended solids, and how resistant microbes are. The method used must allow proper contact time to work well.
Organizations like the EPA and WHO have created safety standards that apply worldwide. Their regulations focus on specific areas: microbial limits, disinfectant residual requirements, and tracking indicator organisms like fecal coliform and E. coli.
Water treatment facilities don’t deal very well with disinfection by-products, which drives new breakthroughs like advanced oxidation processes and immediate monitoring systems. These technological advances help facilities balance effective pathogen removal against chemical risks.
Water disinfection has become a vital public health measure. Water professionals keep adapting their approaches as we learn more about microbial threats and treatment technologies. Careful application of disinfection science and following regulations will give our communities safe water resources. This basic process protects millions every day from disease outbreaks, making it the most essential part of modern water treatment infrastructure.
Key Takeaways
Understanding how water disinfection works is essential for protecting public health and ensuring safe water systems. Here are the critical insights every water treatment professional and stakeholder should know:
• Disinfection serves as the final protective barrier against waterborne pathogens, targeting disease-causing microorganisms rather than achieving complete sterilization like medical equipment requires.
• Three primary methods dominate water treatment: chlorination (most cost-effective with residual protection), UV radiation (chemical-free DNA disruption), and ozonation (powerful oxidation without harmful residuals).
• Multiple factors determine disinfection success: pH levels, temperature, suspended solids, microorganism resistance, and proper contact time all significantly impact treatment effectiveness and must be optimized.
• Regulatory compliance requires strict monitoring of microbial limits (99.9% Giardia removal, 99.99% virus removal), disinfectant residuals (0.2-4.0 mg/L for chlorine), and indicator organisms like E. coli.
• Innovation addresses persistent challenges through advanced oxidation processes that eliminate both pathogens and harmful by-products, plus real-time sensor monitoring for immediate process optimization.
Water disinfection represents one of the most critical public health interventions in modern infrastructure. As treatment technologies advance and regulatory standards evolve, the fundamental goal remains unchanged: protecting communities from waterborne diseases while minimizing chemical risks through scientifically-informed treatment approaches.
Frequently Asked Questions
Q1. What are the main methods used for water disinfection in treatment plants?
The three primary methods used for water disinfection are chlorination, ultraviolet (UV) radiation, and ozonation. Chlorination is the most widely used due to its cost-effectiveness and residual protection. UV radiation uses light to disrupt pathogen DNA without chemicals, while ozonation employs powerful oxidation to destroy microorganisms.
Q2. How does chlorine disinfect water?
Chlorine disinfects water by forming hypochlorous acid and hypochlorite ions when added to water. These compounds penetrate microbial cell membranes and disrupt vital cellular functions, effectively eliminating bacteria and viruses by inactivating essential enzymes that microorganisms need for survival.
Q3. What factors affect the effectiveness of water disinfection?
Several factors influence disinfection effectiveness, including pH levels, temperature, suspended solids, microorganism type and resistance, and contact time. For example, higher temperatures generally increase disinfection efficiency, while suspended solids can shield microorganisms from disinfectants.
Q4. What are disinfection by-products (DBPs) and why are they a concern?
Disinfection by-products are harmful compounds formed when disinfectants react with organic materials in water. Common DBPs include trihalomethanes (THMs) and haloacetic acids (HAAs). They are a concern because long-term exposure has been linked to increased cancer risks and other health issues.
Q5. How do regulatory standards ensure safe drinking water?
Regulatory bodies like the EPA and WHO establish strict standards for water disinfection effectiveness. These include specific inactivation levels for pathogens, disinfectant residual requirements, and monitoring for indicator organisms like fecal coliform and E. coli. Compliance with these standards helps ensure consistent water safety across different treatment facilities and distribution networks.






